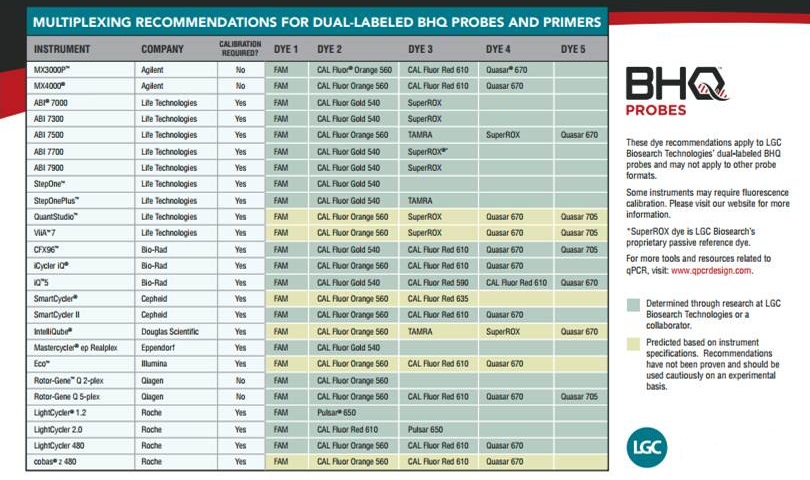
.jpg) Dual-Labeled BHQ Probes are traditional, linear, FRET probes incorporating a fluorophore and quencher covalently attached to the 5' and 3' ends of an oligo typically 20 to 30 bases in length. Fluorescence is released through the 5' exonuclease activity of Taq polymerase, which cleaves off the fluorescent dye upon the probe's hybridization to its complementary sequence. Dual-Labeled BHQ Probes are ideal for detecting the presence and quantifying the amount of specific target sequences.
Dual-Labeled BHQ Probes are traditional, linear, FRET probes incorporating a fluorophore and quencher covalently attached to the 5' and 3' ends of an oligo typically 20 to 30 bases in length. Fluorescence is released through the 5' exonuclease activity of Taq polymerase, which cleaves off the fluorescent dye upon the probe's hybridization to its complementary sequence. Dual-Labeled BHQ Probes are ideal for detecting the presence and quantifying the amount of specific target sequences.
We also offer ValuMix for gene expression and qPCR which delivers one BHQ probe and two primers in a single tube. Valumix primer quantities can be flexibly adjusted around a specified probe amount of either 0.5 nmol, 5 nmol, or 20 nmol delivered. Probe to primer ratios can range anywhere from 1:1 to 1:4.5 according to your preference. ValuMix is an economical option providing 0.5 nmol of FAM-BHQ probe mixed with primers.
Design Concerns for BHQ probes
Please be aware that sequences longer than 30 bases are unlikely to have efficient quenching as an end-labeled probe. For such situations please consider positioning the quencher internally instead. Conversely, sequences shorter than 20 bases may have been designed as a Minor Groove Binder (MGB™) probe and are unlikely to bind at the proper temperature of 70 °C. Please consider ordering such a sequence as a BHQplus® probe.
NOTE:
1. Building Probes with an Internal Quencher (Currently available with FAM only)
2. Adding an internal modification annotated by T(...) indicates that a Thymidine base is attached to the internal modification.
3. Please be aware that an additional T base will be introduced into the sequence when adding such an internal modification.
4. Building Probes with Wobble/Degenerate Bases
5. To ensure the most efficient processing of your oligo, use IUPAC code when entering wobble/degenerate bases in the Sequence Entry field instead of using parentheses, e.g. (A/G), (A/G/C/T), etc.
For Quotations & Order Requests
.jpg) Dual-Labeled BHQ Probes are traditional, linear, FRET probes incorporating a fluorophore and quencher covalently attached to the 5' and 3' ends of an oligo typically 20 to 30 bases in length. Fluorescence is released through the 5' exonuclease activity of Taq polymerase, which cleaves off the fluorescent dye upon the probe's hybridization to its complementary sequence. Dual-Labeled BHQ Probes are ideal for detecting the presence and quantifying the amount of specific target sequences.
Dual-Labeled BHQ Probes are traditional, linear, FRET probes incorporating a fluorophore and quencher covalently attached to the 5' and 3' ends of an oligo typically 20 to 30 bases in length. Fluorescence is released through the 5' exonuclease activity of Taq polymerase, which cleaves off the fluorescent dye upon the probe's hybridization to its complementary sequence. Dual-Labeled BHQ Probes are ideal for detecting the presence and quantifying the amount of specific target sequences..jpg)