Bioanalytics is critical in drug discovery and development. We in collaboration with companies such as GenScript provide a wide range of biophysical, biochemical and functional analysis for comprehensive ab/protein characterizations, using our state-of-art analytical platforms.
With professional scientists and advanced facilities, We provide individual analysis and integrated analytical solutions for clients from lead generation, lead optimization, drug candidates, stable cell line development, all the way up to pilot scale production.
Biochemical Assays:
(a) Affinity ranking/ measurement (Biomolecular Interaction Analysis Services): Surface plasmon resonance (SPR) is an invaluable tool to characterize real-time molecule – molecule interaction such as affinity and kinetics analysis. With strong expertise, extensive experience, proprietary high throughput screening and affinity ranking technology, we are capable of satisfying your demands in SPR services in any type, including affinity ranking, affinity measurement and kinetics measurement. our Biomolecular Interaction Analysis Services deliver analysis data with high accuracy, reasonable price and high speed.
Key Features:
1. Extremely high accuracy by professional operation and strict quality control
2. Unparalleled high-resolution kinetics using BIAcoreT200 with almost no limitation to ligand molecular weight
3. Fast delivery
(b) Epitope mapping
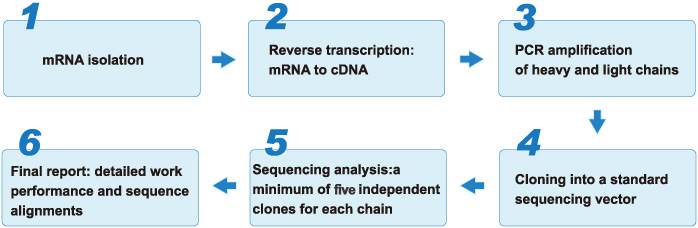
(c) Antibody sequencing: The antibody sequence information is essential for monoclonal antibodies (mAbs) engineering, function optimization, database banking, and patent application. Our antibody sequencing service is your reliable choice to speed up and facilitate therapeutic antibody leads discovery and development. Our services are tailored to meet your particular requirements, option with customized specifications is offered for antibody sequencing services.
Standard Sequencing Procedure
Key Features:
1. Superb accuracy
2. Competitive pricing for high quality
3. Short turn-around time
Cell-based Assays
(a) Immune checkpoint assay: Immune checkpoints represent a group of extracellular membrane-bound proteins expressed on immune effector cells (e.g. T/B cells, NK cells), either inhibiting or stimulating effector cell proliferation. They are involved in eliminating foreign pathogens while maintaining self-tolerance, playing a crucial role in immunomodulation. Nowadays, production of therapeutic antibodies designed to block or activate immune checkpoints has become a new powerful approach for the treatment of cancer and other diseases.
To meet the growing demand of drug discovery on immune checkpoint targets, We constantly explore new technologies for cell-based reporter bioassays with high specificity, sensitivity and reproducibility. The bioassays are complied with ICH guidelines with outstanding performance in antibody screening, characterization, potency testing and stability studies.
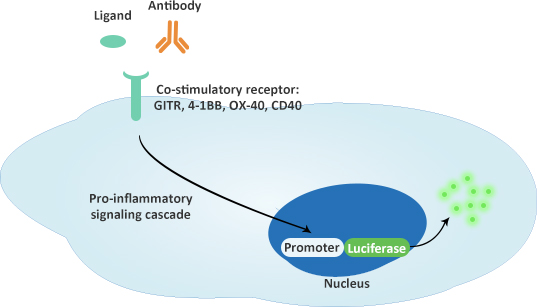
Co-stimulatory Immune Checkpoint Bioassay
Mechanism of action: anti-GITR, anti-4-1BB, anti-OX40 and anti-CD40 antibodies function to mimic the ligand.
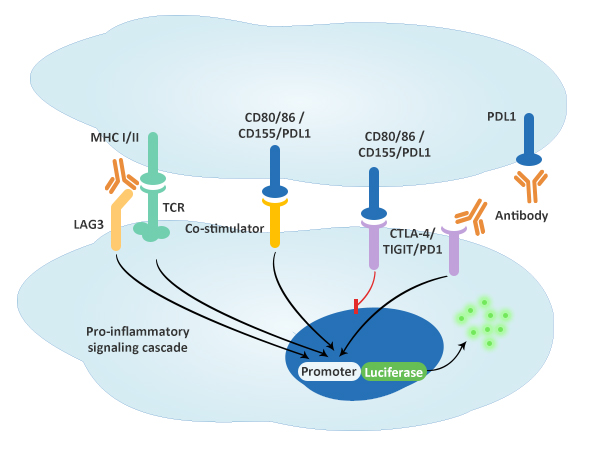
Inhibitory Immune Checkpoint Bioassay
Mechanism of action: anti-CTLA-4, anti-TIGIT, anti-PD1, anti-PDL1 and anti-LAG3 antibodies block CTLA-4, TIGIT, PD1, PDL1 and MHC I/II respectively to activate the downstream production of reporter enzyme.
Key Features:
1. Assay targets: PD-1, PD-L1, CTLA-4, OX-40, CD-40, GITR, 4-1BB, TIGIT, and LAG3
2. Fast turn-around time and robust performance
3. Stringent documentation management, supporting preclinical development
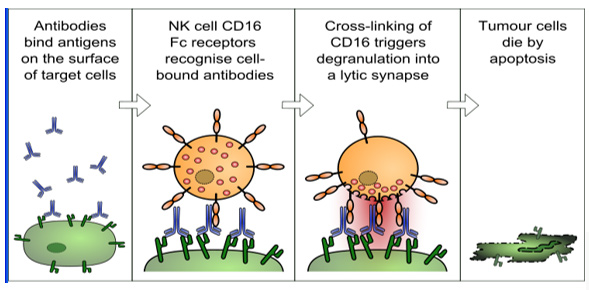
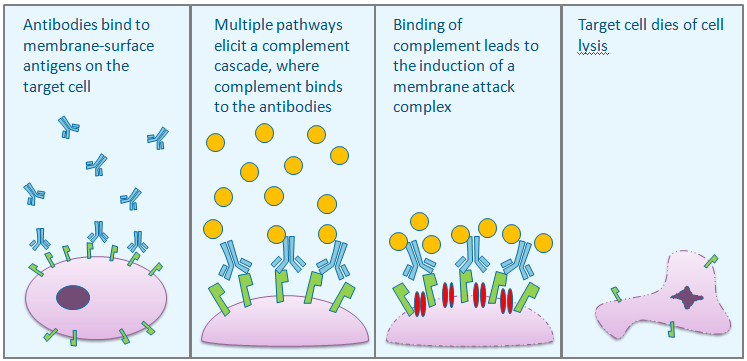
(b) ADCC/CDC/ADCP assay: Antibody therapy has been proven to be highly powerful for cancer treatment. Two important mechanisms used by antibody drugs to kill targeted tumor cells are Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC), and Complement Dependent Cytotoxicity (CDC). We are pleased to present to you both a PBMC-based ADCC assay and natural kill cell-based ADCC assay. The readout is endpoint-driven (target cell lysis). The CDC assay uses normal human serum as the source of complement. By implementing strict QC standards, providing the assurance of the efficacy and potency profiles of your therapeutic antibodies.
ADCC Mechanism
CDC Mechanism
(c) Apoptosis, proliferation, and phagocytosis assay: Oncology drug discovery requires knowing the pharmacological mechanism, and efficacy of small molecules on human tumor cell lines. We offer a comprehensive oncology drug discovery platform which includes antibody drug discovery, in vitro and in vivo pharmacology services.
Key Features:
1. High quality assay criteria and short turnaround time
2. OncoProfiler includes 200 human tumor cell lines.
3. High throughput screening (HTS) options available
(d) Neutralization assay
(e) Assay cell line development: Recombinant stable cell lines are one of the widely used tools in drug discovery, toxicity testing, and basic research. Long-term stable expression of a gene of interest (GOI) is usually achieved by transfection or viral transduction of a vector containing the expression cassette of the GOI together with a selection marker (antibiotics or fluorescent proteins). In contrast to transient expression, stable cell line offers reproducible results for assay studies.
Protein Analytics
(a) Molecular weight analysis
(b) Sequence coverage analysis
(c) PTM analysis
(d) Purity test
(e) pI test
(f) Charge variant analysis
(g) N/C terminal sequence verification
(h) N-glycan analysis
(i) Impurity analysis (HCP,HCD…)
For Quotations & Order Requests